News
Discover all the news from SQS
How to Approach Data Space Projects in Organizations: Interoperability, Governance and Security
February 13, 2026Data Spaces are rapidly emerging as one of the main enablers of new collaboration models between organizations across Europe. Industries...

Data Spaces and Interoperability: Why Technical Validation Will Be Key in the Coming Years
February 10, 2026Data spaces are transforming how public and private organisations share, consume and govern information. Technical interoperability—and above all, the validation...
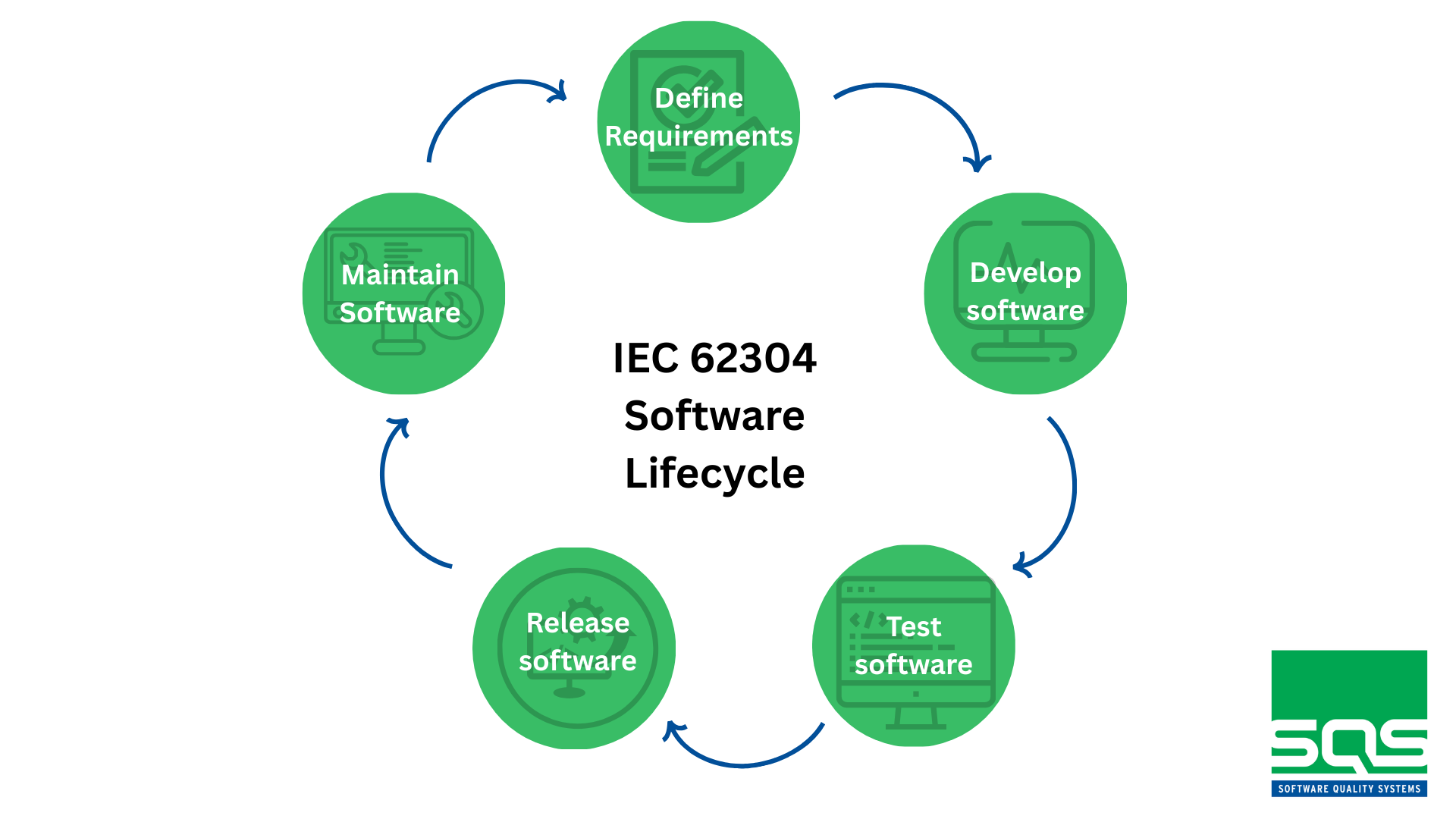
IEC 62304 as a Reference Framework for the Medical Device Software Lifecycle
January 29, 2026Software is at the core of many modern medical devices, from pacemakers to glucose-monitoring applications. Its correct functioning can be the difference...

ENS and Secure Software: Keys to Complying with the Spanish National Security Framework Without Blocking Operations
January 27, 2026How to approach ENS compliance in an agile, practical and business-oriented way More and more public bodies and technology companies...

How to Guarantee Software Quality in Highly Regulated Environments (Railway, Medical Devices, Critical Industry)
January 13, 2026Practical guide based on SQS experience In the most regulated sectors (railway, medical devices, energy, critical industry), software quality is...

Data Spaces and Data Quality: The New Critical Territory for Digital Companies
December 22, 2025Data spaces are redefining how organisations share data securely, governed and in a decentralised way. From mobility to health, energy...

X-Raying the Code: How Static Analysis Ensures the Safety of Medical Software
December 10, 2025The First Shield of Medical Software: Static Analysis According to IEC 62304 In the development of software for medical...

Software Quality in Regulated Environments: Meeting Standards Without Slowing Innovation
December 9, 2025Critical software in regulated sectors requires extremely high levels of quality: reliability, safety, traceability and regulatory compliance. Errors or non-compliance...

Software Test Automation: Why You Must Address It Now (and How to Do It Well)
November 25, 2025Test automation is no longer optional: it is a key piece in the competitiveness of any company that develops or...

POCT and IVDR Regulation: What Really Applies at European and National Level
November 19, 2025Point-of-Care Testing (POCT) refers to diagnostic tests performed near the patient, designed to deliver rapid results without the need for...

IVD Regulation in Spain: Royal Decree 942/2025
November 3, 2025Royal Decree 942/2025 adapts Regulation (EU) 2017/746 for in vitro diagnostics In vitro diagnostic medical devices (IVDs) play an essential...

QA&TEST Embedded 2025: Three Days of Innovation, Inspiration, and Community in Bilbao
October 27, 2025SQS, the organizer of the international conference QA&TEST Embedded, held a new edition from October 22 to 24 at the...

UNE-ISO/IEC 20000-1
UNE-EN ISO/IEC 17025
ENS-nivel alto
Approved IDS Evaluation Facility
Subscribe to our newsletter
Follow us
Disclaimer | Cookies Policy | Code of Ethics | Quality, Safety and Environment Policy | Contact
© 2026 Software Quality Systems S.A. | SQS is a member company of Innovalia




