Nowadays, the manufacturing of pharmaceuticals and medical devices is heavily reliant on automated processes. What would happen if one of these automated processes were to fail? It could potentially affect the quality of the product being manufactured, ultimately posing a risk to the health of those who would use the medication or medical device.
Importance of Regulatory Compliance
As the malfunction of these systems can put people’s health at risk, the validation of computer systems in the pharmaceutical and medical device industry is heavily regulated by organizations such as the FDA (Food and Drug Administration) in the United States and the EMA (European Medicines Agency) in Europe.
Computer Systems Involved in Manufacturing Processes
A classic example of a computer system that requires validation in the pharmaceutical industry is the Laboratory Information Management System, commonly known as LIMS. In the medical device sector, the validation of ERPs (Enterprise Resource Planning systems) is often common, as it usually includes processes related to their manufacturing that, in case of malfunction or misuse, could compromise the quality of the manufactured product.
Computer Software Validation
As mentioned earlier, manufacturing companies are mandatory to validate their computer systems. The ultimate goal of these validations is to ensure the quality, traceability, and data integrity of the products being manufactured. To achieve this, the main activities typically carried out to demonstrate their proper functioning include:
- Validation Planning
- Risk Analysis
- Design and Development
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Traceability Matrix
- Validation Report
Revalidation of Computer Systems
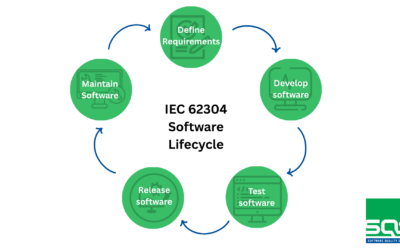
It is essential to recognize that computer systems used in the pharmaceutical and medical device industry must be continuously maintained in a validated state. These systems are subject to frequent updates, either to correct identified errors or to incorporate new features that enhance their performance. Before applying any updates, a thorough assessment of the proposed changes is required to determine their impact on the system. This assessment is critical and must be followed by a rigorous revalidation process to confirm that the updated system continues to operate reliably and meets all established performance criteria, thus ensuring its functionality and compliance with current regulations.
Conclusion
Computer software validation in the pharmaceutical and medical device industry is a fundamental pillar to ensure the safety, efficiency, and quality of medical products. This methodical process not only complies with regulatory requirements but also supports data integrity and ensure confidence in automated systems that play critical roles in production and quality control. Validation is an ongoing cycle that accompanies the system throughout its life, adapting to technological updates and improvements. Effective validation significantly contributes to patient safety and operational efficiency, establishing itself as an indispensable practice in the constant pursuit of excellence in the healthcare industry.
At SQS, we are experts in performing tasks related to computer system validation, from validation planning and risk analysis to the final validation report, tailoring activities to the latest applicable regulations.
Need assistance with the validation of your computer systems?
Contact us