SQS will participate in MEDICA 2025, the leading international trade fair for healthcare technology and medical devices. The event will take place in Düsseldorf, Germany, from November 17 to 20, 2025. SQS will be part of the Spanish delegation organized by FENIN (Spanish Federation of Healthcare Technology Companies), which promotes innovation and international visibility for the national industry.
At SQS, we bring over 20 years of experience in quality software consulting and testing in regulated environments, especially in medical devices and digital health. Our services include:
- Verification and validation of medical software according to MDR, IVDR, and FDA regulations.
- CE marking specialized in software as a medical device.
- Cybersecurity services: Pentesting and implementing a secure software lifecycle for medical applications.
- Computer System Validation: For compliance with ISO 13485 and GxP regulations.
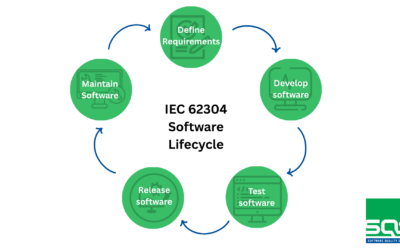
- Implementation of Quality and Security Management Systems: ISO 13485, IEC 62304, IEC 81001-5-1, ENS, and ISO 27001.
Participating in MEDICA reinforces our commitment to ensuring the safety, quality, and regulatory compliance of healthcare software. It also allows us to showcase Spanish innovation on a global stage with over 80,000 industry professionals.
We invite all attendees to visit the Spanish Pavilion at MEDICA 2025 to learn how SQS can help ensure the reliability of software in medical devices and digital health solutions.