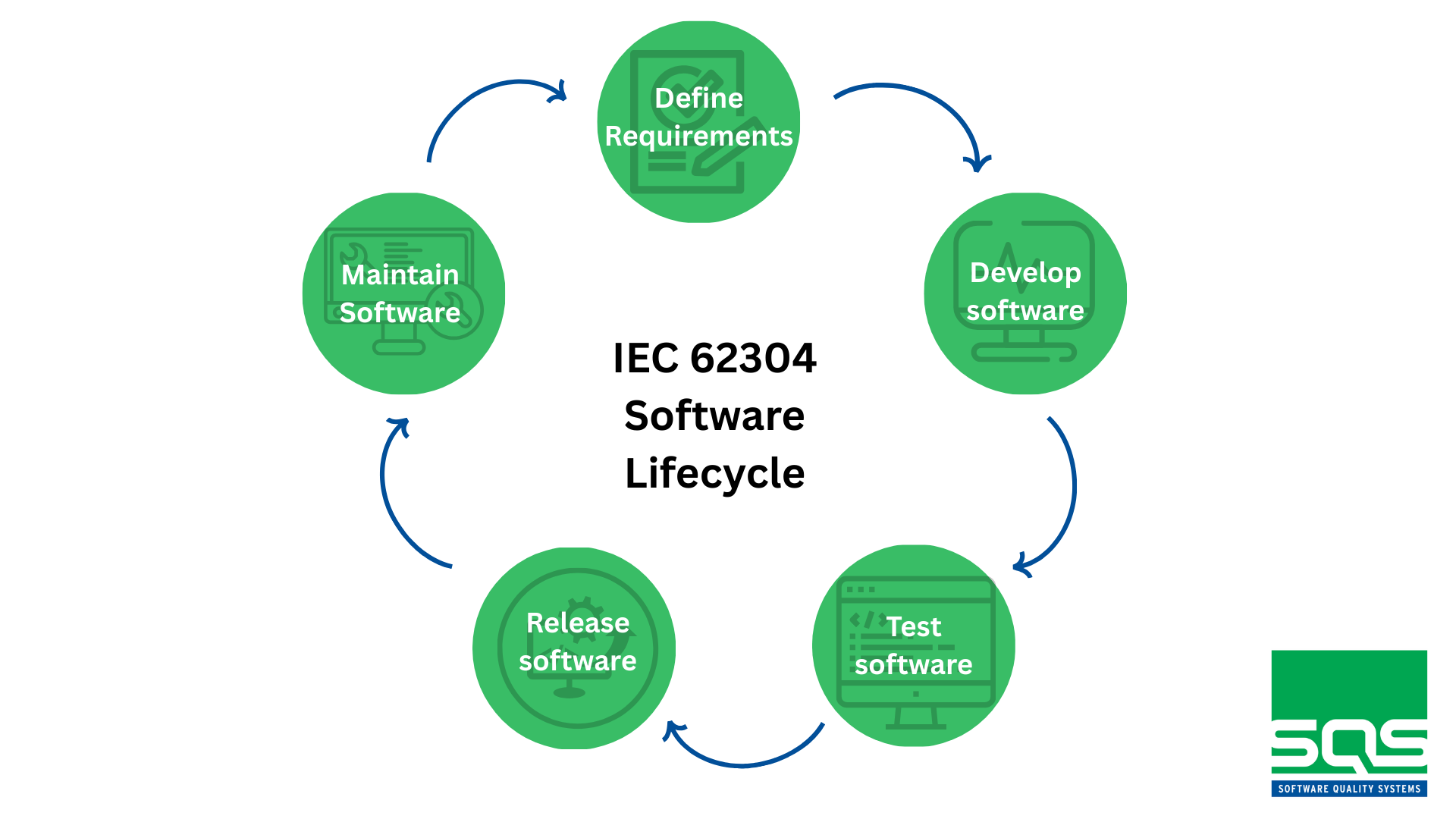
To ensure that medical software is reliable, safe, and aligned with regulatory expectations, the international standard IEC 62304 defines the required processes for the entire software lifecycle. It provides a structured framework for developing, testing, releasing, and maintaining medical device software in a controlled and fully traceable way.
IEC 62304 is the globally recognized reference standard for medical device software and is essential to:
- Obtain CE marking in Europe
- Facilitate FDA clearance in the United States
- Integrate software risk management with ISO 14971
- Demonstrate a consistent, audited lifecycle in compliance with MDR/IVDR
Unlike generic software standards, IEC 62304 focuses specifically on patient safety, traceability, and software risk control.
Key Benefits for Manufacturers
Adhering to IEC 62304 provides clear advantages in both safety and competitiveness:
- Enhanced patient safety, thanks to systematic identification and mitigation of software-related risks
- Regulatory compliance with MDR/IVDR and FDA expectations
- Standardized and repeatable processes for development, verification, validation, and maintenance
- Greater trust from customers, notified bodies, and healthcare professionals
Key Features and Differentiators of the Standard
IEC 62304 introduces several essential aspects that make it a reference framework for the medical industry:
- Software risk classification
The standard defines three software safety classes based on the potential harm in case of failure:
- Class A: Minimal risk
- Class B: Moderate risk
- Class C: Critical risk
For example, a glucose-monitoring software system must undergo extensive testing and full documentation (Class C), while a medical scheduling app follows a simpler process (Class A).
- Fully documented software lifecycle
From requirements definition to maintenance and updates.
- Integrated risk management
Every potential failure is analysed, evaluated, and mitigated following ISO 14971.
- Formal verification and validation
Evidence-based testing proportional to the software safety class, with full traceability between requirements, design, tests, results, and anomalies.
- Software Maintenance and Problem Resolution
A core part of IEC 62304 is ensuring that safety is maintained after release. The standard requires:
- A documented maintenance process
- Analysis and categorization of field issues and bugs
- Risk-based evaluation of corrections
- Full traceability of updates, patches, and design changes
- Structured problem resolution activities aligned with ISO 14971
This ensures that software continues to perform safely throughout its lifetime and that all updates remain compliant.
How IEC 62304 Drives the Medical Technology Industry
Proper application of IEC 62304 accelerates regulatory approval and shortens audit times, reducing legal exposure and the likelihood of recalls.
It also ensures higher patient safety and confidence, strengthening manufacturers’ reputation as leaders in safe medical software development.
How SQS Can Help You
At Software Quality Systems (SQS), we support healthcare and medical device companies in implementing IEC 62304 efficiently and pragmatically, aligning the regulatory baseline with their existing quality processes and ensuring compliance with the EU MDR and U.S. FDA requirements.
Our specialized services include:
✅ IEC 62304 compliance assessment
✅ Definition and implementation of the IEC 62304-compliant software lifecycle
✅ Verification, validation (V&V), and risk-management support
✅ Integration with ISO 13485 and ISO 14971
✅ Training and support during audits and certification processes
With extensive experience in the medical device sector, we help organizations reduce compliance time and costs without compromising quality or safety.
IEC 62304 is more than a regulatory requirement—it is the foundation for secure, reliable, and globally compliant medical device software.
Adopting this standard means committing to excellence, patient safety, and responsible innovation.
💡 SQS helps you implement IEC 62304 in a practical and efficient way, adapted to your company and your processes.
Contact us to learn how we can support you in validating and ensuring regulatory compliance for your medical device software.